*OS=overall survival.

It’s Time to Take Them All On
You do everything in your power to maximize overall survival (OS). It’s a measure that may depend on durability of effect and staying on therapy. In addition, other measures like progression-free survival (PFS), objective response rate (ORR), disease etiology, quality of life (QOL), preserving liver function, bleeding risk, and treatment discontinuation rates all need to be addressed effectively, too.
Liver cancer:
A growing health problem in the United States1
new cases of liver cancer are estimated in 20232
Incidence of HCC tripled in the last decade5
NASH is the fastest growing cause of HCC, particularly in the West6
NASH=nonalcoholic steatohepatitis.
Hepatocellular carcinoma:
The most common type of primary liver cancer7
Despite progress in the treatment of unresectable or metastatic hepatocellular carcinoma (uHCC), existing therapy options leave room for improvement
TKI monotherapy—first introduced more than a decade ago—continues to be prescribed as 1L therapy despite availability of newer regimens.
~1 in 5
patients with advanced HCC received 1L TKI therapy12
…and many patients go untreated…
44%
of patients with advanced HCC received no systemic therapy
37%
of those who did receive 1L therapy, received no subsequent therapy13
Data from US community clinics N=586
…including in intermediate-stage HCC…
46%
of intermediate and advanced uHCC patients received no active treatment (defined as LRT/TACE ± systemic therapy)14
Cancer registry and health claims data N=215
…and for those in treatment, Off-target inhibition with cancer treatments may lead to discontinuation DUE TO ADVERSE EVENTS (AEs)15
LRT=locoregional therapy; TACE=trans-arterial chemoembolization.
While early 1L uHCC treatment used monotherapy – primarily with tyrosine kinase inhibitors (TKIs) – more recent options focus on a combination therapeutic approach16
Overall survival (OS)
—the ultimate measure of efficacy—
mOS peaked at 19.2 months in clinical trials among existing uHCC combination therapies17
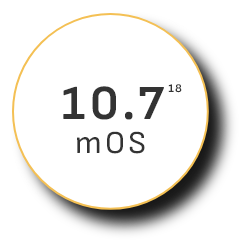
Monotherapy 2007
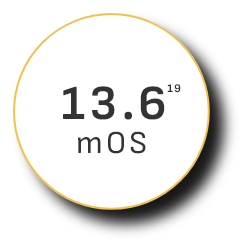
Monotherapy 2015
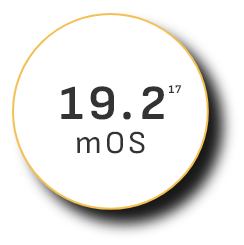
PD-1 + VEGF therapy 2020
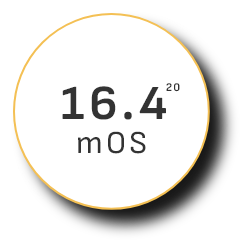
PD-1 + CTLA-4 therapy 2022
Progression-free survival among existing therapies
ranges from 3.8 to 7.3 months19,20
Median PFS
Monotherapy
2007
Monotherapy
2015
PD-1 + VEGF therapy
2020
PD-1 + CTLA-4 therapy
2022
*Median PFS not tracked. This number indicates time to tumor progression.
CTLA-4=cytotoxic T-lymphocyte antigen 4; PD-1=programmed cell death protein 1; PFS=progression-free survival, defined by the FDA as time from randomization until objective tumor progression or death, whichever occurs first; VEGF=vascular endothelial growth factor.
Objective response rate (ORR) has been as high as 28% and as low as 19% among existing uHCC treatments19,22
ORR
Monotherapy
2007
Monotherapy
2015
PD-1 + VEGF therapy
2020
PD-1 + CTLA-4 therapy
2022
CTLA-4=cytotoxic T-lymphocyte antigen 4; PD-1=programmed cell death protein 1;
VEGF=vascular endothelial growth factor.
Quality of life (QOL)
Patients with uHCC have significantly impaired quality of life. With regard to QOL, there are two main treatment goals. The first is to minimize the impact of the disease itself on the patient. The second is to minimize the negative impact that treatment may have on patient QOL due to adverse events. Factors that may impair QOL include physical functioning, GI symptoms, pain, and fatigue. These factors must be taken into consideration when deciding on a treatment path to help preserve QOL.16,22,23

Liver function can complicate treatment options
Patients with uHCC should be routinely evaluated as treatment options can be impacted by the presence of underlying liver disease. Both Child-Pugh and albumin-bilirubin (ALBI) measures can be used to assess liver function in patients with uHCC.
Asymptomatic patients may often present with late-stage disease that can complicate or limit potential treatment options. Liver function at the time of clinical presentation is recognized as an important prognostic factor in uHCC patients. Studies have shown that more severe liver dysfunction is associated with increased overall mortality.24-27
Hemorrhage risk
The study of one 1L combination therapy showed the most common AEs leading to discontinuation were hemorrhages. Those consisted of gastrointestinal, subarachnoid, and pulmonary hemorrhages.
The study also showed gastrointestinal and esophageal hemorrhage leading to death. Depending on therapy, patients may require prior screening for varices. This could potentially delay treatment initiation.22

Discontinuation rate due to AEs
Discontinuation rates due to adverse events (AEs) have been as low as 9% and as high as 32%18,22
Monotherapy
2007
Monotherapy
2015
PD-1 + VEGF therapy
2020
PD-1 + CTLA-4 therapy
2022
CTLA-4=cytotoxic T-lymphocyte antigen 4; PD-1=programmed cell death protein 1; VEGF=vascular endothelial growth factor.
Stay Connected
Sign up to receive information, company news and updates, or to connect with a member of our team.
REFERENCES: 1. Su GL, Altayar O, O’Shea R, et al. Gastroenterology. 2022;162(3):920-934. 2. Siegel RL, Miller KD, Sandeep Wagle N, Jemal A. CA Cancer J Clin. 2023;73:17-48. 3. Sadhu A, Blandy O. Decisions Resources Group. Published November 20, 2020. 4. Sadeghi S, Bejjani A, Finn RS. Surg Oncol Clin N Am. 2019;28(4):695-715. 5. Weinfurtner K, Dodge JL, Yao FYK, Mehta N. Transplant Direct. 2020;6(10):e605. 6. Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Cell Metab. 2022;34(7):969-977. 7. Golabi P, Fazel S, Otgonsuren M, Sayiner M, Locklear CT, Younossi ZM. Medicine (Baltimore). 2017;96(9):e5904. 8. Cidon EU. World J Hepatol. 2017;9(18):797-807. 9. Eatrides J, Wang E, Kothari N, Kim R. Cancer Control. 2017;24(3):1-9. 10. Rawla P, Sunkara T, Muralidharan P, Raj JP. Contemp Oncol (Pozn). 2018;22(3):141-150. 11. Tarao K, Nozaki A, Ikeda T, et al. Cancer Med. 2019;8(3):1054-1065. 12. Data on file. Elevar Therapeutics; 2023. 13. Gupta A, Girvan AC, Sheffield KM, et al. 2021 ASCO GI 2021. Abstract 279. 14. Shankaran V, Chennupati S, Sanchez H, et al. J Hepatocell Carcinoma. 2021;8:1597-1606. 15. Lin A, Giuliano CJ, Palladino A, et al. Sci Transl Med. 2019;11(509):eaaw8412. 16. Kang D, Shim S, Cho J, Lim HK. Korean J Radiol. 2020;21(6):633-646. 17. Finn RS, Ikeda M, Zhu AX, et al. Cancer Res. 2021;81(13 Suppl):CT009. 18. NEXAVAR. Prescribing information. Bayer HealthCare Pharmaceuticals Inc.; July 2020. 19. LENVIMA. Prescribing information. Eisai Inc.; November 2022. 20. IMFINZI. Prescribing information. AstraZeneca Pharmaceuticals LP; November 2022. 21. Llovet JM, Ricci S, Mazzaferro V, et al. N Engl J Med. 2008;359(4):378-390. 22. TECENTRIQ. Prescribing information. Genentech USA, Inc.; December 2022. 23. Hengrui Clinical Study Report. A Randomized, Open-Label, International, Multi-Center, Phase 3 Clinical Study of PD-1 Antibody SHR-1210 plus Apatinib (Rivoceranib) Mesylate versus Sorafinib as First-Line Therapy in Subjects with Advanced Hepatocellular Carcinoma (HCC) Who Have Not Previously Received Systemic Therapy. Jiangsu Hengrui Pharmaceuticals Co., Ltd.; 2023:1-293. 24. NCCN. Clinical Practice Guidelines in Oncology. Hepatocellular Carcinoma, version 1.2023. Accessed July 26, 2023. 25. Tsoris A, Marlar CA. Use Of The Child Pugh Score In Liver Disease. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK542308/ 26. Toyoda H, Johnson PJ. JHEP Rep. 2022;4(10):100557. 27. Massarweh NN, El-Serag HB. Cancer Control. 2017;24(3):1073274817729245.





